The emergence of Coronavirus and Diagnosis
Coronavirus disease (COVID-19) has emerged as that superstar who needs no introduction. Coronam is the Latin term for crown, and coronavirus has the crown-like appearance. As of now, global Covid-19 deaths have exceeded over 8 lakhs with total cases at 25.1 million and still counting. There was only one lab for testing on January 23, 160 labs on March 23 and 1370 labs by August, in India according to the health ministry.
Previously known as a 2019-novel coronavirus (2019-nCoV) was officially renamed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by International Committee on Taxonomy of Viruses (ICTV) on February 11, 2020. This name was selected because the virus is genetically related (70% similarity) to the coronavirus responsible for the SARS outbreak of 2002. Diseases are officially named by WHO in the International Classification of Diseases (ICD) while viruses are named by the ICTV.
Diagnosis of COVID-19 involves multiple phases from sample collection to submitting data of individuals in the ICMR portal.
Sample collection-
Most commonly, nasal and throat swabs are collected and transported to the nearest testing facility in the Viral transport medium. Each individual is assigned a unique Specimen Referral Form (SRF) ID at the time of sample collection, and details of the individual are recorded.
 |
| Samples are received in the above cooling boxes |
Labelling and neutralization-
Once the samples reach their destined labs, they are allotted lab IDs which are meant to easily distinguish among different samples. Further, neutralization is carried in Biosafety cabinet (BSL-2 and above required), during which virus-cell lysis is done using lysis buffer. This procedure requires maximum preventive measures, as mishandling could result in virus spread. Post neutralization, samples are sent for RNA isolation.
 |
| Post neutralization remaining sample volume is stored at -80 ֯ C |
RNA isolation-
Coronaviruses are a large family and its genetic material is a single-stranded positive-sense RNA (+ssRNA); thus, RNA isolation is must to detect its presence in an infected individual. This RNA is then converted to complementary DNA (cDNA), using an enzyme called reverse transcriptase and PCR is done. Internal controls are used as an indicator of perfect nucleic acid extraction, quality of samples, quality of PCR.
For a quicker screening of individuals pooled sample approach for RNA extraction is being employed. Under this approach, samples from individuals (3 or 5 samples are generally pooled into 1) are mixed into a single tube and RNA extraction proceeds.
If a pool appears negative for E-gene after RT-PCR (Real-time PCR), then all individuals are considered negative. However, if a pool is detected positive then, RNA extraction is individually done for the pooled samples and tested for E-gene as well as RdRp gene (RNA-dependent RNA polymerase). RdRp is essential for the replication of the virus genetic material once it infects a host. Thus, individuals are diagnosed positive or negative based on the results obtained from two different genes.
Real-time PCR and data analysis-
In real-time PCR, a positive reaction is determined by the accumulation of a fluorescent signal. Generally, real-time assays undergo 40 cycles of amplification. The cycle at which the fluorescent signal can be detected is termed cycle quantification (Cq) value.
Some instruments show a cycle threshold (Ct) value instead, which is the number of cycles required for the fluorescent signal to cross the threshold value. Not to be confused, both the Cq and Ct values are technically similar. These values show how many cycles it took to detect a real signal from samples.
CY5 (cyanine5) is a synthetic dye which detects the internal control and thus confirms the quality of RNA and PCR. If Cq or Ct value for CY5 dye is nil, then the RNA extraction should be repeated. The other dye FAM (fluorescein amidite) is specific for coronavirus specific gene called E-gene. E-gene encodes a small membrane protein (E protein), necessary for the assembly of virions.
If a person tests positive, then there must be a Cq value for FAM (values greater than 36 are considered as false positives while between 22 to 36 are considered positive), while a person negative for the presence of the virus has no Cq value for FAM. Similarly, FAM is also capable to detect RdRp gene, which is another coronavirus specific gene. Furthermore, positive and negative controls provided with the real-time kit are also run simultaneously to verify the assay.
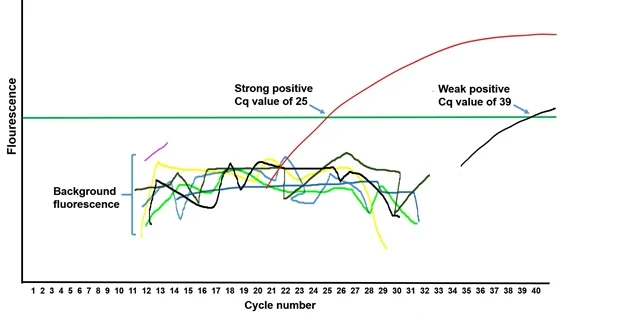 |
| Real-time PCR amplification curve showing Cq value of different samples |
The relation between Cq value and amount of target gene-
Cq values are inverse to the amount of the target gene (here E and RdRp gene) expression in samples. Lower Cq values indicate high amounts of target sequence while higher Cq values correspond to weak expression of the target gene.
Other commonly available methods for testing
Rapid diagnostic tests based on antigen detection–
Rapid antigen tests account for about 30% of the total Covid-19 test conducted in India. The rapid test requires sampling similar for a molecular test (real-time PCR), but detects viral proteins (antigens) on the surface of the virus instead of genetic material and also provide results within 10-15 minutes.
However, antigen tests are not as sensitive as molecular tests but are useful due to their quicker output. Rapid antigen tests have been found to provide more false negatives and intermittent false positives. Due to these irregularities in the results, ICMR (Indian Council of Medical Research) has issued an advisory on antigen detection test, individuals who detect negative must be tested successively by RT-PCR to rule out infection, while a positive test should be considered true positive if the symptoms of the patients also, confirm so and does not require confirmation by RT-PCR.
Rapid Antibody detection tests–
Antibody detection test is different from the other diagnostic tests, as it identifies people who were infected and have recovered. Unlike RT-PCR or antigen test, antibody detection requires a blood sample.
It depends on the detection of antibodies against SARS-CoV2 in a blood sample, which can be easily obtained through a simple finger prick.
Two different antibody isotypes, IgM and IgG are detected by this test. If a person has a recent exposure to the virus, then IgM (Immunoglobulin M) antibodies are detected. While the detection of IgG indicates a later stage infection. If both IgG and IgM antibodies are displayed in the test, then the transformation from IgM to IgG humoral response must have been initiated.
Antibody response requires several days to develop; thus, this test may not be beneficial in determining the current infection status.
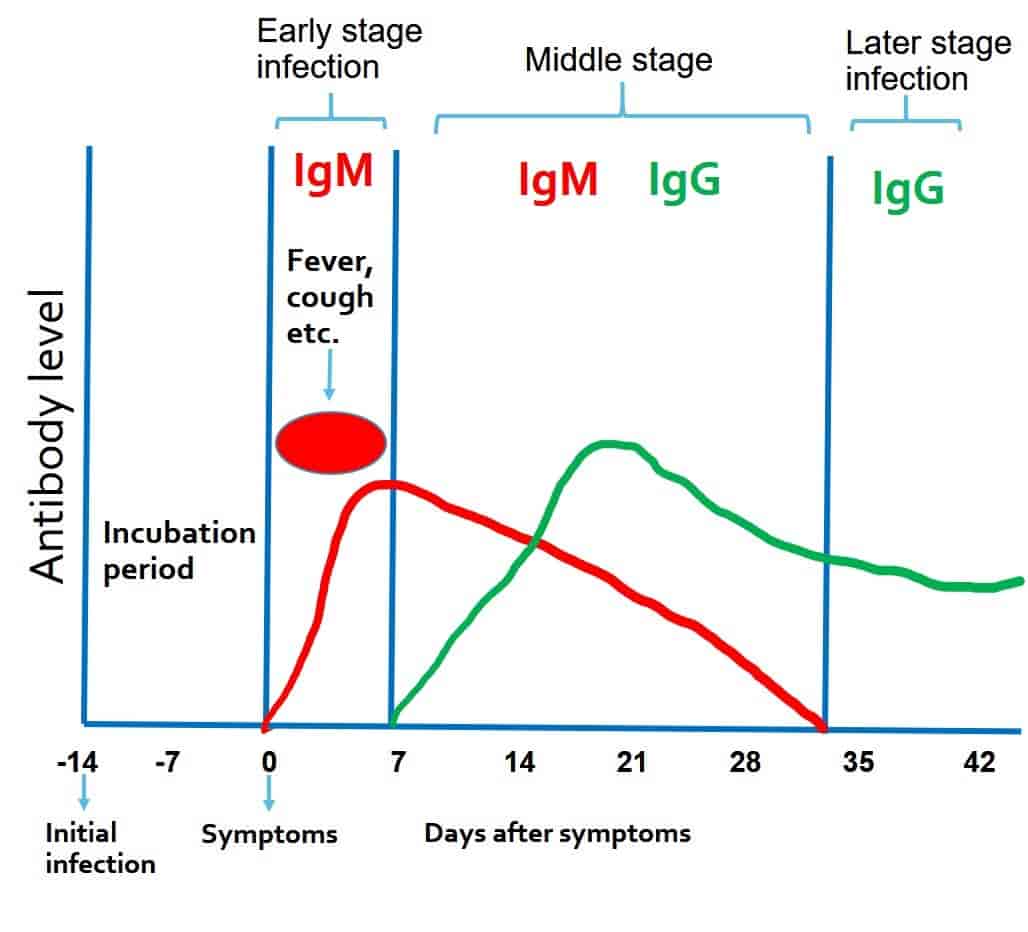 |
| Antibody response against Covid-19 |
The principle behind antibody test-
IgM antibodies are the first antibodies which are produced initially in response against the virus, therefore indicate a recently initiated infection. While IgG antibodies have a higher affinity to bind with the target antigen (here SARS-CoV2), thus are generated at the later stage of the disease.
Technically, an antibody test is similar to the test done for pregnancy detection, which also utilizes a chromatographic immunoassay.
How long does it require to test covid positive after contacting a positive patient?
It may take about 3-4 weeks for a person to test positive for an antibody test as it detects the antibodies developed by an individual in their blood. However, an RT-PCR test can detect positive results within 10-14 days after the infection.
Reinfection of Coronavirus
According to the world health organization, it was unclear if being infected meant one can’t be re-infected again. However, recent reports have shown re-infection of patients with COVID-19 from Hongkong, Belgium and Netherland. Thus, precautionary measures are must at these challenging times.



