Hemocytometer Calculator
Table of Contents
Protocol for counting cells using hemocytometer calculator
Counting cells during a regular passage or for experimental application:
1. Proceed based on whether the cell is suspended or adherent. Centrifuge cells at 220 xg for 5 min. Aspirate most of the supernatant, except for 100-200 μl to avoid disturbing pellet.
2. Re-suspend cell pellet in 1 mL medium, or more if large pellet, but keep track of what volume you use to re-suspend your cells.
3. Take 100 uL sample of re-suspended cells and add to Eppendorf tube.
4. Add 100 uL of Trypan blue to Eppendorf tube and pipette up and down a few times to ensure an even suspension. Gently pipette solution into each well of the hemocytometer in order to fill the area under the coverslip by capillary action.
(It is important not to disturb the coverslip in this process, to maintain an even distance between the glass and the hemocytometer grid. This ensures that each large square contains 0.0001 mL or 0.1 µL of suspension).
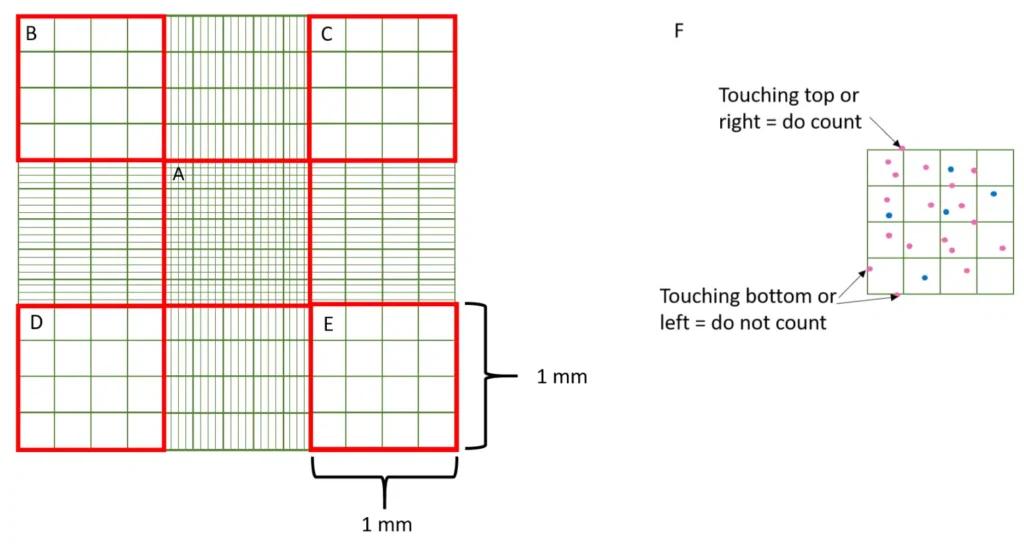
5. Count the number of dead and alive cells in 5 of the large squares of the hemocytometer grid, using phase contrast microscope under ~10x power.
(For standardization, count those in the 4 corner squares and center square. For cells touching an edge, count the ones touching the top and right edges and exclude those touching bottom and left edges)
6. Dead cells appear dark/blue, as dye enters through cell membrane. Live cells appear more clear centrally.
7. Now, just input the values in our provided hemocytometer calculator and see the detailed results and the formula used in your calculation.
Watch & Learn- How to use a hemocytometer? 🎥
Check out the below ⬇️ video for a step-by-step guide on using a hemocytometer.
Mathematical formulas for hemocytometer calculation
- Viable Cell Concentration (cells/ml) = Average number of viable cells per square x Dilution factor x 10⁴
If you are wondering 🤔 where did 10⁴ came from, here is the explanation below.
We multiply by 10,000 because the hemocytometer only allows us to count cells in a very small volume (0.1 µL or 0.0001 mL per large square). To express the concentration in a standard unit (cells per mL), we scale up by: 10,000. This accounts for the fact that there are 10,000 of these 0.0001 mL volumes in 1 mL, ensuring the final result represents the total number of cells per milliliter (0.0001ml x 10,000 = 1ml).

2. Dead Cell Concentration (cells/ml) = Average number of dead cells per square x Dilution factor x 10⁴
3. Dilution factor = Total volume after adding 0.2% trypan blue dye (µL) ÷ Initial volume of cell suspension (µL)
4. Total number of Live Cells in Stock Solution = Concentration of live cells (cells/ml) x Total volume of stock solution (ml)
5. Total number of Dead Cells in Stock Solution = Concentration of dead cells (cells/ml) x Total volume of stock solution (ml)
6. Percentage Cell Viability Calculator = (Number of Live Cells ÷ Total Number of Cells ) x100
7. Percentage Cell death Calculator = (Number of Dead Cells ÷ Total Number of Cells ) x100
Try solving below: Question & Solution Inside! 🚀
Question: You are a researcher tasked with the setting up a series of experiments and analysing the resultant data from a study of the effects of the organophosphate chlorpyrifos on the viability and differentiation of cultured mouse N2a neuroblastoma cell line, as indicated below. You are required to handle, analyse, present and interpret the data provided, providing evidence of how you have arrived at your conclusions Cells were grown as a mitotic monolayer to approximately 80% confluence then detached for counting. After centrifugation of the resultant cell suspension, cell pellets were suspended in 1 mL growth medium. A volume of 10 µL of this cell suspension was mixed with 90 µL growth medium containing 0.2 % v/v Trypan Blue, after which the cells analysed in a haemocytometer, giving the data shown in Table 1.
| Square number* | Total cell count | Blue (dead) cell count |
| 1 | 99 | 3 |
| 2 | 115 | 8 |
| 3 | 87 | 6 |
| 4 | 105 | 5 |
| 5 | 125 | 9 |
*Each haemocytometer square has a volume of 10-4 ml. Using the data in Table 1, calculate the following parameters.
(i) Calculate total cell count/ml
(ii) Viable cell count/ml
(iii) % Viability
Solution:
Answer (i) Total cell count/mL means we have to calculate total concentration of live and dead cells.
Total cell count/mL = Average of total cells per square x dilution factor x 104
Average of total cells per square= Total cells counted ÷ Total number of squares counted
Average of total cells per square= (99+ 115 + 87 + 105 + 125) ÷ 5= 106.2
Dilution factor= 100 ÷ 10= 10
Total cell count/mL = 106.2 x 10 x 104 = 1.062 x 107 =10,620,000 cells/mL = 10.62 million cells/mL
Answer (ii) Viable cell count/mL = Average viable count per square x dilution factor x 104
For each square, calculate the number of viable cells = Total count – blue cell count
Average viable count per square = Total viable cells counted ÷ Total number of squares counted
Average viable count per square = (96+107+81+100+116) ÷ 5 = 100
Viable cell count/ml = Average viable count per square x dilution factor x 104
Viable cell count/ml = 100 x 10 x 104 = 1 x 10⁷ viable cells/mL = 10 million viable cells/mL
Answer (iii) % Viability= (Total number of viable cells per ml ÷ Total cell count per ml) x 100
% Viability= (10 million ÷ 10.62 million) x 100 = 94.2%
OR
% Viability= (Average viable count per square ÷ Average of total cells per square) x 100
% Viability= (100 ÷ 106.2) x 100 = 94.2%
